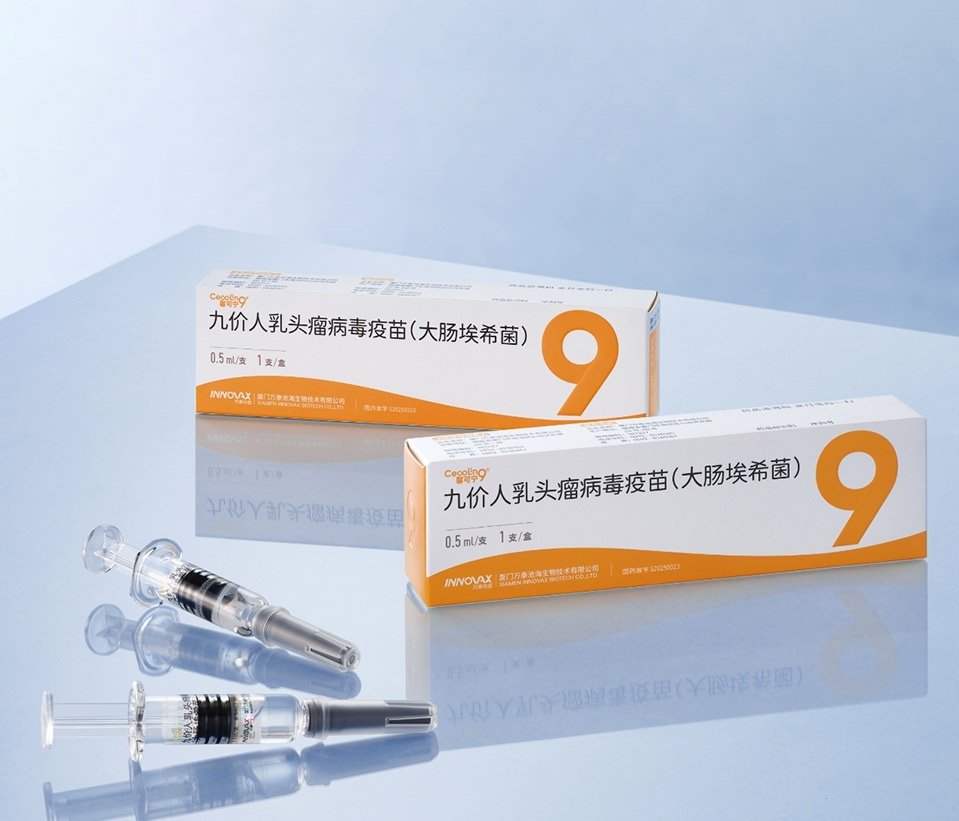
In 2025, China's National Medical Products Administration (NMPA) approved Cecolin®9, the first HPV 9-valent vaccine in developing counrtries originated from China. As the second HPV 9-valent vaccine available worldwide, it breaks the long-standing global technological monopoly held by imported high-valency HPV vaccines. This milestone marks China as the second nation (after the United States) with complete, independent capabilities to sustainable supply of a high-valency HPV vaccine.
Building on Cecolin®'s proven efficacy against HPV 16/18 (responsible for cervical lesions and cervical cancers), Cecolin®9 derived from the same trusted platform with expanded protection capabilities by covering 5 additional high-risk types (31, 33, 45, 52, 58) and 2 low-risk types 6/11(preventing genital warts and related conditions).
Cecolin® 9 embrace independent intellectual property covering 9 types HPV VLP, featuring the groundbreaking truncated HPV16 L1 protein technology—honored with China's Patent Gold Award (the nation's highest IP distinction), along with Special Prizes from both Fujian Province and Xiamen City. This R&D achievement was supported by China's National 863 Program and recognized by the Gates Foundation.

International Quality Standards: Cecolin®9 was developed in accordance with European Union (EU) quality standards. Comparative studies have demonstrated that its immunogenicity and safety are equivalent to those of international counterparts. These findings were published in The Lancet Infectious Diseases. Notably, the research conclusion that "the domestically developed 9-valent HPV vaccine shows comparable efficacy to imported vaccines" was selected as one of "China's Top Ten Medical Science News of 2023."
Robust Clinical Data: Cecolin®9 demonstrates robust clinical validation through five nationwide trials since 2019 and enrolled over 11,000 healthy female volunteers aged 9-45. A large-scale randomized, controlled Phase III clinical trial in women aged 18–45 showed: (1) excellent efficacy against HPV16/18 infections/lesions, (2) >98% protection against persistent infections (≥12 months) from HPV31/33/45/52/58, including 100% cervical infection prevention, and (3) consistently favorable safety throughout observation.
Flexible Immunization Schedule: 2 doses for females aged 9-17 years, 3 doses for females aged 18-45 years