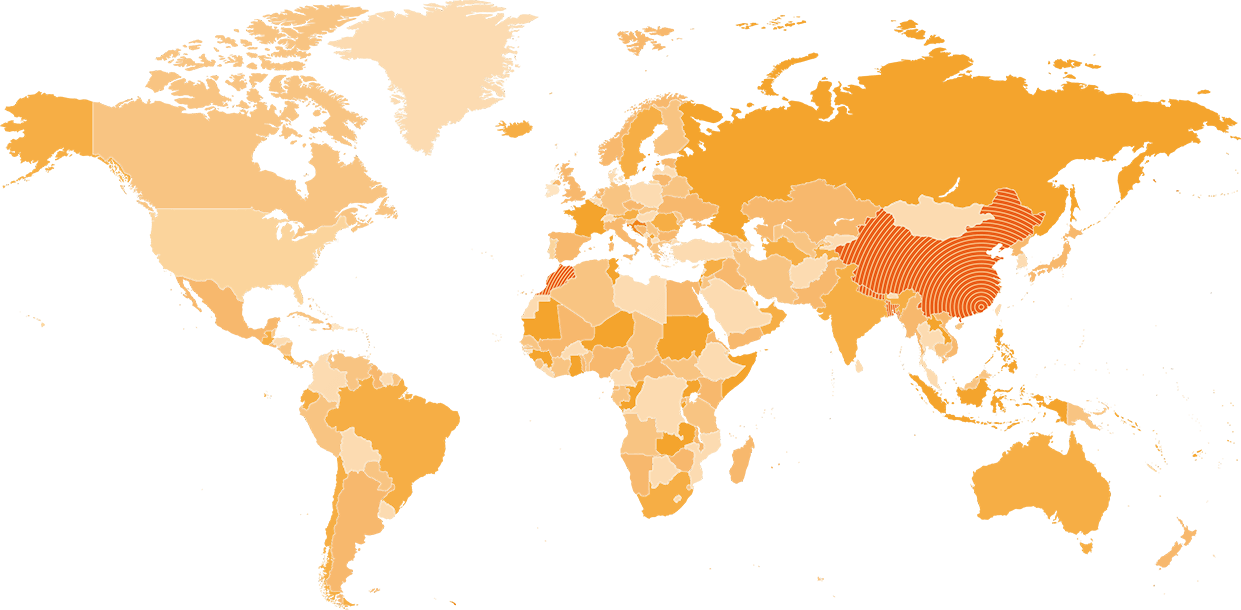
China’s first HPV vaccine, Cecolin® was approved by the National Medical Products Administration on December 30, 2019, China becomes the third country in the world that rely on its self-supply of HPV vaccines, after the United States and the United Kingdom.
With a cumulative investment of about RMB 800 million in 16 years, Cecolin® gained support from the National Science and Technology Major Project (on the innovation and manufacturing of new drugs), 863 Program and other International Cooperation Projects.


HPV 16 and 18 are two high-risk viruses responsible for most cervical cancers, which are the target of Cecolin® . Unique advantages enable Cecolin® to become a powerful weapon for the world to eliminate cervical cancer and to meet the global planned immunization needs.
Being China's first HPV vaccine that only two doses are required for women aged 9-14 years;
The protective efficacy against CIN2/3, AIS or cervical cancer associated with HPV-16 and/or 18 was 100%.
The efficacy in preventing infection with HPV 16 and/or 18 for over 6 months was 97.7%, for over 12 months was 95.3%.
Featuring lower price and the widest applicable age range (9 to 45 years old)